Chlorination- Residual Chlorine
Chlorination is the most widely recognized term utilized while managing water treatment processes. Most water treatment plants are expected to sanitize the water of their utilization by the interaction: of chlorination, to kill hurtful microscopic organisms. The most often utilized technique for sterilization is the expansion of chlorine. Chlorine sanitizers give a “lingering” level of insurance against waterborne microorganisms. A chlorine lingering is a low degree of chlorine staying in water after its underlying application. It comprises significant protection against the gamble of resulting microbial defilement after treatment – a novel and huge advantage for general well-being.
Cl2 + H2O —-> HOCl + H+ + OCl–
Chlorine dose
How much chlorine is added to the water for chlorination intention is known as the chlorine portion. This is a deliberate amount picked by the administrator and brought into the water utilizing a chlorinator or hypo chlorinator. At the point when chlorine is added to water, a portion of the chlorine responds first with natural materials and metals in the water. What’s more, it became inaccessible for sterilization (this is known as the chlorine interest of the water). The excess chlorine fixation after the chlorine request is represented is called all-out chlorine leftover. All out chlorine is additionally separated into:
- The amount of chlorine that has reacted with ammonia or other nitrogen-containing organic substances in the water is called combined chlorine
- Free chlorine, is the chlorine available to inactivate disease-causing organisms.

(Chlorine demand) = (Chlorine dose) – (Total Chlorine residual)
(Total Chlorine residual) = (Free Chlorine) + (Combined Chlorine)
The graph below shows what happens when chlorine (either chlorine gas or a hypochlorite) is added to water in the chlorination process. First (between points 1 and 2), the water reacts with reducing compounds in the water, such as hydrogen sulfide, and Fe2+. These compounds use up the chlorine, producing no chlorine residual
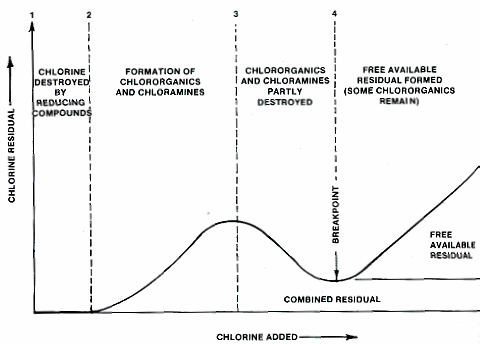
Next, between points 2 and 3, chlorine reacts with organics and ammonia naturally found in the water. Some combined chlorine residual is formed – chloramines.
Monochloramine:
NH3 + HOCl —> NH2Cl + H2O
Dichloramine:
NH2Cl + HOCl ——> NHCl2 + H2O
Trichloramine:
NHCl2 + HOCl ——> NCl3 + H2O
The experimental process to find out the dose of chlorine, Social and environmental impacts is available here.

Hi, I am John Smit a Captain in Fire Department City of Newyork with over years of experience in the field of Firefighting and HSE. My passion for fire safety started when I was a young boy and witnessed a neighbor’s house go up in flames along with precious lives. Since then, I had dedicated my life to ensuring the safety of buildings, properties, and individuals in case of a fire and medical emergencies.




