
What is an Ozone Layer?
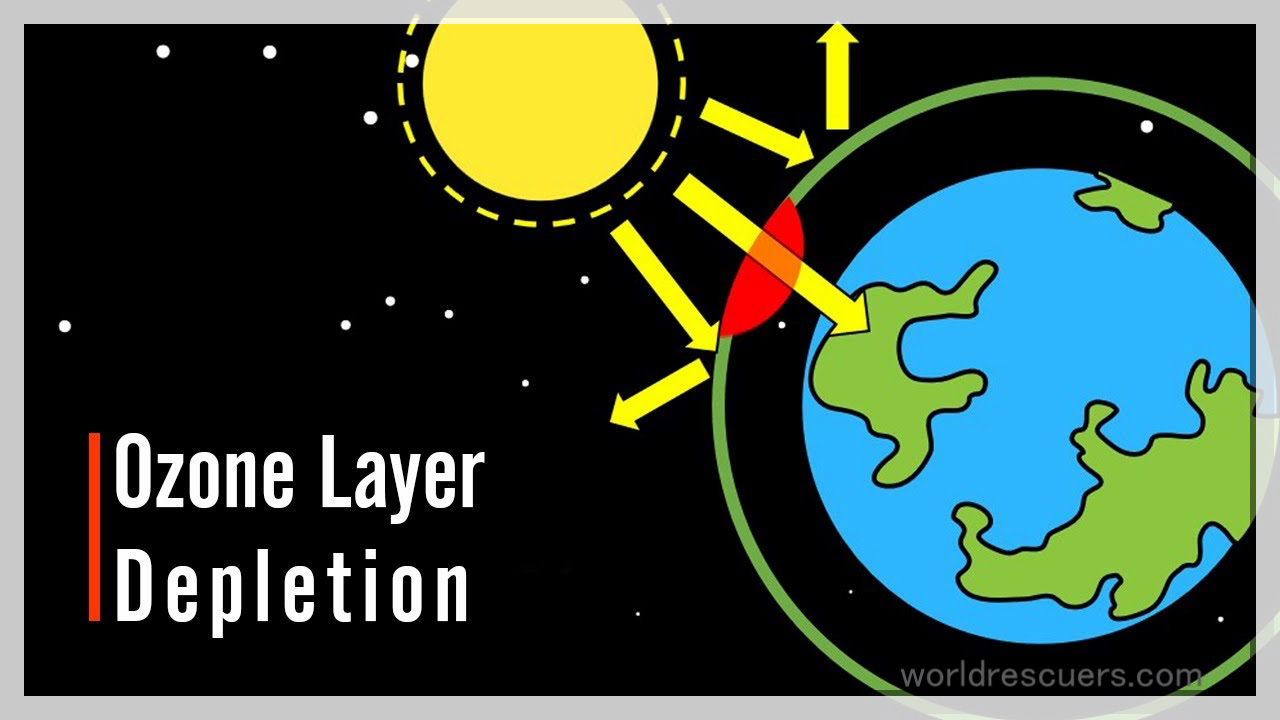
Have you ever wondered why cricketers paint faces white? Or why your mother insists that you put on sunscreen lotions before stepping out every summer? The answer is UV rays or ultraviolet rays. UV rays are harmful sun rays that can increase the risk of skin cancer, and cataract and harm the immune system. They can also damage to rest your plant lives, single-cell organisms, and equated ecosystems. Life on Earth is protected from the UV rays by a layer in the stratosphere and environment called the ozone layer.
Ozone is a gas made up of three oxygen atoms. This layer is just about three to 5 mm thick and this thinly spread out gas has been protecting life on earth’s surface from UV rays for billions of years. Our ozone shape is now being deteriorated due to certain manmade chemicals, primarily chlorofluorocarbons, CFCs and nitrogen oxides. CFCs are a group of chemicals. Similar gases are used in refrigeration systems, air conditioners, aerosol solvents, and in the production of some types of packaging. Nitrogen oxides are a byproduct of fuel burning, for example from aircraft exhaust. What is the ozone hole?
The ozone hole is not a hole, but an area where the total ozone amount is less than 220 Dobson units. The ozone hole has steadily grown in size up to 27 million km². Can we stop the depletion of the ozone layer? Yes, we can. All we have to do is to reduce the production of those chemicals that destroy ozone like CFCs and nitrogen oxides. So encourage your parents, relatives, and friends to make sure their refrigerators and air conditioners do not have CFCs.
Depletion And Its Effects
These holes in my blanket. Hey there. Gets quite cold out here. But this blanket is a savior, did you know? So just like this blanket saves us from the cold, our planet also has its blanket called the ozone layer. What does the ozone layer save us from, though the sun, which is a source of life on Earth, also sends some harmful radiations away, called ultraviolet or UV radiation? Since the ozone layer is so important, we need to keep it safe. And we also need to make sure that there is no hole in the ozone layer. But is there a hole in the ozone layer? My blanket is made up of cotton mostly.
To find out more about the holes in the ozone blanket, let’s find out what it’s made up of. Now, the ozone blanket is made up of oxygen atoms. But oxygen atoms can group as molecules in two or three to form different molecules. Hello, friend. Hi. Yeah, the oxygen molecule is our friend, without whom we can’t live. Even the oxygen molecule is made up when two atoms of oxygen stay together.

Now, these oxygen molecules are from the oxygen gas that we breathe in all the time. Well, the ozone layer is kind of the party zone for the oxygen, if you will, higher up in the sky. I mean, in the upper layers of the atmosphere. Not two, but three oxygen atoms come together with the help of sunlight. Now, these three oxygen atoms, right together form the ozone molecule, and these ozone molecules form the ozone blanket or the ozone layer. However, it’s a good thing that the ozone layer has its zone high up.
Why did you ask?
Because if these ozone molecules came down nearer to us, we could breathe it in just like we would breathe oxygen. And that can damage our lungs, can cause cancer, and even threaten our life. It can also impact evaporation rates, cloud formation, and precipitation levels of a place and cause global warming. So many differences in behavior. This trio, as compared to this duo inviting ozone nearer to us, is a big no. The ozone is a friend. But far away, right so far away in the sky, the ozone molecules absorb the harmful radiations, the ultraviolet radiations from the sun, protecting life on Earth.
Now, the ozone layer keeps life on Earth safe from harm, just as my blanket keeps me warm. But can you guess how thick this safety ozone blanket might be? Well, if we collect all the ozone from different heights in the atmosphere into a single blanket, it would be even thinner than this blanket of mine. Only about three to five millimeters more power to a special blanket. But this raises a serious question, right? If there is a hole in the ozone layer, will the sun’s harmful radiation just reach us and come and harm us directly? Well, thankfully, the ozone hole is a hole. As such, not a hole like it is in my blanket.
What Environmental Scientists did observe?
Well, back in the 1980s, environmental scientists did observe that the ozone layer was becoming thinner and thinner over the Antarctic regions, more likely painted patches or you might find on an old blanket. Now, this hinting meant that the number of ozone molecules substantially decreased in that region. This Ozone Layer Depletion was named the ozone hole simply because it appeared like a hole in the colored images from satellite data. However, it alerted us to the threat to the ozone, and scientists set out to find the enemies of our far-away friends and protect us.
The search for ozone’s enemies led to chemicals called chlorofluorocarbons PFCs. But where do these monstrous chemicals that kill our ozone come from? Now, the sad thing is, I can say that the hole in this blanket of mine was because of rats or because I was not careful. But as for the ozone hole, we humans are to be blamed. These chemicals that harm the ozone were at least from appliances like refrigerators and air conditioners and fire extinguishers, which were commonly used in industries and homes.
Ask a Question
You might ask a question our ozone friends are so far away, they stay so high up away from us. Yet how do these chemicals that we produce around us on Earth manage to harm the ozone? This is the bad news. These chemicals do not die easily. They remain in the atmosphere for hundreds of years. This gives them enough time to reach the ozone layer and kill the ozone molecules. Do you know why? The ozone hole was specifically observed over some regions, like the polar regions on Earth. Now, that’s because these regions are cold. And cold or low temperatures help the CFCs in killing the ozone molecules. We had to save the ozone to save life on Earth. And the solution was the Montreal Protocol, signed by 28 countries in the year.
To regulate the emission of harmful chemicals and gases in the atmosphere. Appliances emitting CFCs were replaced by more ozone-friendly substitutes. So what happened after that for our ozone friends in the ozone layer? As the harmful chemicals became less and less, the oxygen atoms powered by energy from the sun started making new friends with each other again. New ozone molecules were born.
Do you know what that means? Ozone started healing itself. But the ozone zone is not just ozone. It’s our zone. It’s our zone of protection. We need to protect it as well. Let’s make sure that we use products that are free from harmful chemicals and gases. Now, while the ozone can heal itself, I am going to need some extra help with this blanket of mine. I’ll deal with it later. By the way, do you know what the word ozone is derived from? It’s derived from the Greek word ozien, meaning smell.
Role of The Rescue 1122 in Improving the Environment.
The Rescue 1122 Services to improve the environment in Pakistan are not hidden. Millions of plants are planted and cared for annually by rescuers. The Clean and Green world campaign is the recommended campaign for all the world which will reduce Ozone Layer Depletion.


Hi, I am John Smit a Captain in Fire Department City of Newyork with over years of experience in the field of Firefighting and HSE. My passion for fire safety started when I was a young boy and witnessed a neighbor’s house go up in flames along with precious lives. Since then, I had dedicated my life to ensuring the safety of buildings, properties, and individuals in case of a fire and medical emergencies.






